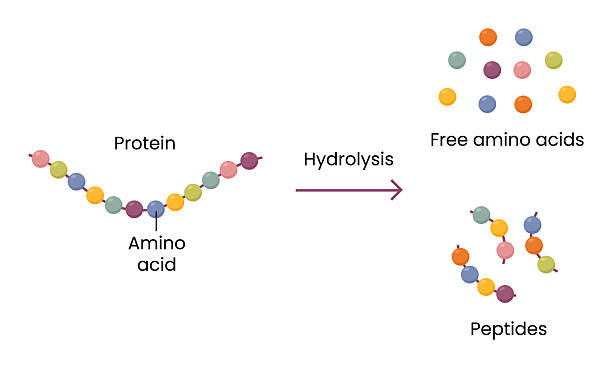
In both chemistry and biology, the reaction known as hydrolysis is essential for many processes that keep life and technology working smoothly. But what is meant by hydrolysis? In simple terms, hydrolysis refers to a chemical reaction in which a molecule breaks apart when it reacts with water. It is a vital mechanism that enables the breakdown of complex molecules into smaller units by adding water. Whether in digestion, manufacturing, or environmental systems, hydrolysis plays a central role.
This article dives deeply into what hydrolysis means, how it works, the various types, where it is found, and its real‑world importance.
Understanding What is Meant by Hydrolysis
Hydrolysis is derived from Greek: “hydro” meaning water and “lysis” meaning to break or split. When water molecules interact with another substance, they cause specific chemical bonds to break, resulting in new products. This process isn’t simply mixing with water — it’s a chemical transformation where water participates directly in breaking the compound.
For example, proteins in food are too large for the body to absorb. Hydrolysis reactions break those proteins into amino acids, enabling absorption. This concept — using water to split complex molecules — is the core meaning of hydrolysis.
How Hydrolysis Works: The Chemical Mechanism
To understand scientifically, we must look at the steps involved:
Water Activation: A water molecule approaches a target compound.
Bond Interaction: Specific bonds within the compound, typically between carbon and another atom (like oxygen or nitrogen), begin to stretch when interacting with water.
Bond Breakage: The bond breaks, and the water molecule donates a hydrogen ion (H⁺) and a hydroxide ion (OH⁻) to different parts of the original molecule.
Resulting Products: Two or more smaller compounds are formed with new chemical structures.
For example, when an ester undergoes hydrolysis, the products are an alcohol and a carboxylic acid. Essentially, water becomes part of the products, illustrating how hydrolysis uses water to split bonds.
Types of Hydrolysis Reactions
Hydrolysis doesn’t occur the same way in every situation. There are several distinct types depending on the chemical environment and the catalysts involved:
Acid Hydrolysis
Acid hydrolysis happens when a strong acid (like hydrochloric acid) accelerates the breakdown of a compound by water. This type is often used in laboratory settings and in the digestive process of carbohydrates.
Example:
Polysaccharides (like starch) + Water + Acid → Simple sugars
Acid hydrolysis is used in food chemistry to break down complex carbohydrates into sweet, simpler sugars.
Base Hydrolysis
Also called alkaline , this reaction occurs when a strong base (such as sodium hydroxide) aids the breakdown of compounds. In organic chemistry, base hydrolysis is crucial in breaking down fats or esters into alcohol and soap — a process known as saponification.
Example:
Fat + Water + Base → Glycerol + Fatty Acids (soap)
This demonstrates how impacts everyday products like soap.
Enzymatic Hydrolysis
In living organisms, often requires enzymes — biological catalysts that speed up reactions without being consumed. Enzymatic is critical in the digestion and metabolism of nutrients.
Proteins → Amino acids (via proteases)
Carbohydrates → Simple sugars (via amylases)
Fats → Fatty acids and glycerol (via lipases)
This type of is precise and controlled, happening at body‑compatible temperatures and pH levels.
Where Hydrolysis Occurs: Everyday Examples
Understanding becomes much easier when you see it in action. is present in nature, industry, and daily life:
1. Human Digestion
When you eat food, reactions powered by enzymes break down large food molecules into smaller units the body can absorb. Without , proteins, fats, and carbohydrates couldn’t be efficiently digested.
2. Soap Making (Saponification)
When animal fats or vegetable oils react with a strong base, produces glycerol and soap. This reaction has been known for centuries and is still used in modern soap production.
3. Environmental Processes
Many plastics and waste materials degrade over time because water molecules cause of their chemical bonds. This slow breakdown helps reduce environmental pollutants.
4. Biotechnology and Pharmaceuticals
Hydrolysis is used to change or activate molecules in drug manufacturing or biotechnology research. Enzymatic hydrolysis is especially important in producing bioactive compounds.
Factors Influencing Hydrolysis
The rate and success of reactions depend on multiple variables:
Temperature: Higher temperatures generally increase reaction speed.
pH Level: Acidic or basic conditions can dramatically influence the reaction.
Catalysts: Enzymes or chemical catalysts make faster and more efficient.
Exposure to Water: The greater the amount of water, the more likely will occur.
Because involves water directly in the chemical transformation, these factors matter greatly in determining how and when it happens.
Hydrolysis in Industrial and Scientific Applications
Understanding helps explain why it’s used in many industries:
Biofuel Production
Hydrolysis breaks down complex plant materials into sugars, which are then fermented to produce ethanol.
Food Processing
Acid or enzymatic improves food texture, flavor, and digestibility by breaking down starches and proteins.
Chemical Laboratories
Researchers use to isolate specific parts of complex molecules or to study molecular structures.
Misconceptions About Hydrolysis
Because the idea involves water and breaking molecules, some people think is simply water dissolving something. It isn’t — is a chemical reaction where water participates in breaking bonds and becomes part of the products.
Another misconception is that only happens in labs. In reality, it occurs constantly — in oceans, soil, our bodies, waste systems, and even washing machines.
Conclusion
So, Hydrolysis is a chemical reaction in which water breaks a compound into smaller parts. This process is vital across biology, chemistry, industry, and everyday life. From breaking down food in our digestive system to creating soaps, biofuels, and environmental degradation of materials, is everywhere.
Understanding helps explain how large molecules become smaller, absorbable, or useful units. It also shows how water — a simple molecule — plays a powerful role in chemical transformations that sustain life and modern technology.
Frequently Asked Questions (FAQs)
1. What is hydrolysis in simple words?
Hydrolysis is a chemical reaction where water helps break a molecule into smaller parts.
2. Where does hydrolysis happen in nature?
It occurs in digestion, soil and water environments, breakdown of plastics, and within living cells.
3. Is hydrolysis the same as hydration?
No. Hydration involves adding water to a molecule without breaking bonds, while hydrolysis uses water to break bonds.
4. What are common types of hydrolysis?
The main types are acid hydrolysis, base hydrolysis, and enzymatic hydrolysis.
5. Why is hydrolysis important in biology?
Because it enables the breakdown of proteins, fats, and carbohydrates into molecules the body can absorb and use.